
Since the industrial revolution, a substantial percentage of our society relies on energy sources to carry out daily activities. Though energy can now come from renewable sources (e.g., wind, hydro, solar, etc.), the most common way of obtaining energy is through the burning of fossil fuels (e.g., gasoline, coal, oil, and natural gas) in combustion reactions resulting in the production of carbon dioxide, a powerful heat-trapping greenhouse gas (Ophardt, 2000). Greenhouse gases are needed to keep Earth’s atmosphere’s temperature balanced, but if excess gases accumulate in the atmosphere then it increases the temperature of Earth. Since the industrial revolution, increase in human activities have led to exceedingly large carbon dioxide emissions which is now accumulating in our atmosphere warming the planet rapidly. Models have shown that if steps towards climate change are not taken, the Earth could warm up to 2 degrees Celsius which will negatively affect Earth life to a great extent (IPCC, 2013).
While there are different options to obtain energy sources, some of them have harmful effects to our environment. One of the most popular ways to obtain energy is through the burning of coal. Coal based energy production accounts for more than 48% of domestic energy generation in the United States (Bligen, 2014 p. 893). The coal industry in the United States produced 782.4 million tons of coal in 2016 (EIA, 2017, page vii). From mining, to transportation to electricity generation, coal releases a lot of toxic pollutants into the air, water and land. The detrimental effects of coal use range from water pollution to health risks but the broader problem scientists observe is the impact to climate change due to the substantial carbon dioxide emissions. Coal-fired power plants are responsible for one-third of America’s carbon dioxide emissions-about the same as all transportation sources–cars, SUVs, trucks, buses, planes, ships and trains–combined (EPA, 2017, page ES-11). Coal is an important source of energy but it adds a significant amount of carbon dioxide per unit of heat energy more than the combustion of any other fossil fuel. In fact, coal combustion emits more than twice the climate changing carbon dioxide per unit of energy than natural gas production (EIA, 2017, Table #1).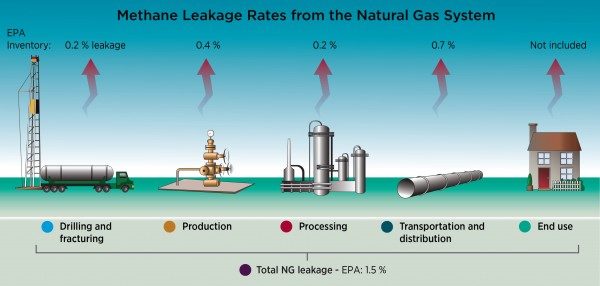
At one point in their lifetime, the average American has used oil as an energy source, indirectly or directly. In addition to coal, the burning of oil has a large impact on our environment. About 40% of the energy consumed in the United States is supplied by petroleum (Bligen, 2010, p. 893). Since the amount of petroleum used varies depending on economics, politics and technology, estimates of carbon dioxide emissions are difficult to predict with certainty. Nevertheless, data has shown that the amount of carbon dioxide released from burning gasoline and diesel fuel was equal to 30% of total U.S. energy-related carbon dioxide emissions (EIA, 2017). In addition to CO2, oil powered plants can also emit particulates NOx and SO2 which are strong gases with direct impact to public health. The economic impact of emissions from oil combustion to public health, including illnesses, premature mortality, workdays lost and direct costs to the healthcare system is equal to 13 cents per kWh (Machol & Rizk, 2013, p. 76).
Since energy is essential for modern economic and social development, it is crucial that the energy sector look for processes that reduce the negative impacts to our climate. Due to the increased concern over carbon dioxide emissions, natural gas production has increased over the past decade. Natural gas, a combustible gaseous mixture of methane and other hydrocarbons, is used extensively in residential energy; more than half of American use gas for home heating. Natural gas is seen as more climatically beneficial and energy efficient than coal or oil because its combustion produces more energy per carbon dioxide molecule formed than coal (170%) and oil (140%) (Karion et al., 2013, p. 4393).
Conventional natural gas extraction involves retrieving gas from large pools by using natural pressure from wells to pump the gas to the surface (British Columbia). However, conventional gas reservoirs have been depleting, therefore the industry relies on unconventional methods to extract gas from shale rock formations. Unlike conventional gas, shale gas remains trapped the original rock that formed from the sedimentary deposition of mud, silt, clay, and organic matter on the floors of shallow seas (UCS). Methods of extracting said gas include horizontal drilling and hydraulic fracturing. Hydraulic fracturing, commonly known as fracking, is a process which is used to create cracks in shale rocks to allow air flow.
The rise of shale gas development can be traced back to the 1840s but the first experiment labeled as hydraulic fracturing occurred by late 1940s. By the 1960s companies such as Pan American Petroleum commercialized these techniques. In 1975, former president Gerald Ford promoted the development of shale oil resources as part of the overall energy plan to reduce foreign energy imports (Manfreda, 2015). The increase cost and climatic disadvantages that the oil and coal industry pose led to the sudden boom in the hydraulic fracturing industry. In 2000 shale gas represented 2% of United States natural gas production. By the end of 2016, it topped 60% (Brown, 2014, page 121; EIA, 2017)
Moreover, hydraulic fracturing also poses advantages to the economy in the United States. On average, the cost of gas extracted using hydraulic fracturing is two to three American dollars per thousand cubic feet of gas. This is 50-66 percent cheaper than production from other energy industries (Sovacool, 2014, page 253). Since conventional gas extractions have become more difficult because of depleting sources, natural gas prices could be 2.5 times higher in 30 years if unconventional gas extractions didn’t exist (Jacoby et al., 2012, p. 46). In addition, shale gas development has been proven to increase employment, revenue and taxes in production areas. Production on the Marcellus Shale brought 4.8 billion US dollars in gross regional product, created 57,000 jobs, and generated $1.7 billion in local, state and federal tax collections (Sovacool, 2014, p. 254). These benefits have prompted the United States to promote hydraulic fracturing as the new standard in the energy industry.
The process of hydraulic fracturing is presented to give a better understanding of how hydraulic fracturing works. The first step in hydraulic fracturing is finding a location with a shale rock formation that will produce natural gas. A shale rock formation is made up of fine grade sedimentary rocks that are are compressed into a clay, the shale that is used in fracking is black shale that is rich in organic matter. The organic matter will undergo heat and pressure and some of it will transform into natural gas. Once the location is found the drilling begins. The drilling is broken into two parts the vertical drilling and the horizontal drilling. The workers first have to drill vertically to a depth around 1,000 feet underground when this is finished a steel casing is inserted into the well so the risk of pollutants won’t spread through the earth’s bedrock and won’t affect groundwater. After the vertical drilling is complete, the horizontal drilling extends out to about 1.5 kilometers through the shale rock formation. After the drilling of the well is completed a specialize performing gunshot is shot which in return creates small holes in the shale formation completing the drilling part of the well (Nacamulli, 2017).
Contrary to popular belief, hydraulic fracturing is not the process of drilling but rather a method used to extract gas after a hole is completed. It is a process that involves injecting water, sand and chemicals at a high pressure into a tight rock formation via a well to stimulate and boost gas flow (Schneising et al., 2014). The propellant in the liquid then goes into the small fractures which keeps them open and allows either the gas or oil to escape from the earth and go up the well and be collected (Schneising et al., 2014). After a well is drilled liquids, such as water and acid, and sand are pumped down the well at high pressures to crack rocks and stimulate shale gas flow. After the shale rock is cracked, the liquid is pumped back to the surface to retrieve the natural gas, this process is known as flowback (Allen et al. 2013). After natural gas is retrieved, the fracking liquid is either pumped back into a separate well and then the well is closed; transported to a water treatment facility or re-used for the stimulation of another well. Recycling the same chemicals with fluid used in new operations contaminates the fluid and creates a more harmful emission the next time around (Nacamulli, 2017). The last step of hydraulic fracturing is the abandonment and plugging of the well. This is done by plugging the well with cement.
While natural gas does decrease carbon dioxide when used as fuel, there is a concern that the process of fracking leads to massive methane escapes, which is concerning since methane is a potent greenhouse gas (GHG). GHGs are gases that trap heat in the atmosphere. GHGs from human activities are the most significant driver of observed climate change since the mid-20th century (IPCC, 2013). The problem lies in the concentration of greenhouse gases in our atmosphere; if too much is in our atmosphere, then more heat is trapped which leads to the planet warming at an unbalanced state. Models have shown that if society doesn’t take the necessary precautions to reduce greenhouse gas emissions, the Earth could warm up by 2 degrees Celsius which substantially impact Earth life as we know it (IPCC, 2013).
As mentioned before, methane is potent strong greenhouse gas with severe environmental impacts; it has a global warming potential (GWP) of 34 (IPCC, 2013). GWP for a gas is a measure of the total energy a gas absorbs over a particular time period compared to carbon dioxide. The larger the GWP, the more warming the gas causes. Methane has a GWP of 34 meaning that it will cause more warming than carbon dioxide. Methane, however, has a shorter life-time in the atmosphere compared to carbon dioxide. Atmospheric lifetime refers to the amount of time a gas stays in the atmosphere before it is released into space. Methane stays in the atmosphere for a decade, carbon dioxide however is more difficult to measure because there is a myriad of biological processes that remove carbon dioxide from the atmosphere therefore carbon dioxide can actually stay in the atmosphere for thousands of years. Carbon dioxide is the focus on climate change reform because of its long atmospheric lifetime but some scientists claim that there is no way to reduce carbon dioxide emissions in time. Even with major carbon dioxide reductions, Howarth argues that the planet could reach 1.5 degrees in 12 years and 2 degrees in 35 years (as cited in Maggill, 2016). Since the planet responds much more rapidly to methane, a reduction in methane emissions could potentially slow global warming. In order for hydraulic fracturing to provide a net climatic benefit, methane emissions must be lower than 3.2% (Alvarez, Pacala, Winebrake, Chameides, and Hamburg, 2012, page 6437. However, studies have shown that methane emissions from operating shale gas formations emit higher percentages of methane than 3.2% (Alvarez et al., 2012; Caulton et al., 2014 ; Karion et al., 2013; Schneising et al., 2014). Methane emissions will continue to increase as fracking grows in popularity therefore reform in technologies need to be made in order to create cost and climatic benefits in energy production.
While fugitive methane leakages at fracturing sites are a recognized concern for climate change, methane emissions and leakage are challenges because they occur at various locations during gas extraction and processing. During flowback, we experience the largest amount of methane emissions are exhibited. As the fracking liquid comes back to the surface, it brings methane released from the shale. During the flowback period, as much as 3.2% of the total natural gas extracted is emitted into the atmosphere (Howarth, Santoro & Ingraffea, 2011 , p. 681). The methane is either captured by emission control devices or emitted into the atmosphere (Allen et al. 2013). Research has shown that methane emissions from shale gas development might be a result of drilling through coal beds which are known to release large amounts of methane. Popular fracking sites, such as the Marcellus Shale formation, are located over coal beds. Another way methane can leak into the atmosphere is through the transportation of natural gas. As natural gas is transported from the well to the storage containers methane leaks through equipment, typically wells have 55 to 150 connections to equipment and make up nearly 90% of methane emission from heaters, meters dehydrators, compressors and vapor-recovery apparatus. (Howarth et al., 2011, Pg. 683) Researchers observed this by examining the unaccounted gas, which is measured by comparing the volume of gas at the wellhead and the amount of gas that was purchased. The estimate of leakage during this time is estimated at 2.5% of emissions (Howarth et al., 2011 Pg. 684-685). Even though it is difficult to trace methane leakage from hydraulic fracturing to just one stage, all of these leaks could be reduced by improving the equipment used. Research performed has shown that the cement used to prevent leaks from well equipments into the atmosphere fails due to installation and material problems (Ingraffea, Wells, Santoro, & Shonkoff, 2014). Since methane emissions from hydraulic fracturing need to be lower than 3.2%, it is crucial that the industry implement reforms to innovate fracking equipment.
Fortunately, methane leaks from fracking are not impossible to stop, and some states have already implemented stricter regulations in order to minimize them. In 2014 Colorado became the first state in the country to place limits on methane emissions from oil and gas operations (Ogburn et al., 2014). Most methane that is lost from fracking comes from leaks in the well infrastructure as well as leaks in the transportation process. In an effort to reduce methane emissions from fracking, Colorado adopted rules which required operators detect and fix leaks and install devices to capture 95 percent of methane emissions (Marmaduke, 2016). It was believed that nearly every step of the methane harvesting process resulted in some amount of methane leakage. In 2016 the Environmental Protection Agency (EPA) passed a rule that was based off of the rule that Colorado had already passed two years earlier. The EPA estimates that theses rules will cut methane emissions by 510,000 tons by the year 2025, which is equal to the amount of greenhouse gases generated by 11 coal fired power plants (Marmaduke, 2016). In the state of Colorado alone, the chief of health estimated that the new rules could cut overall air pollution by 92,000 tons, which is the equivalent of taking every car in the state of Colorado off the road for an entire year (Kroh, 2013). Colorado made significant changes to their emissions standards by requiring all fracking companies to install maximum achievable control technology (MACT). MACT is a set of standards set by the EPA for over 100 categories of different sources of air pollution (West Virginia, 2014). This means that for each of the sources of the pollution the EPA has observed they have set a standard for that source that the company needs to meet. In most cases these standards involve having to install new equipment and machinery that allows less leaks (West Virginia, 2014). In order to install the MACT technology, oil and gas companies will need to be prepared to devote serious financial resources to making it happen. Implementing MACT would force companies to upgrade technology by installing pollution controls, including activated carbon injection, scrubbers or dry sorbent injection, and upgrade particulate controls (Bipartisan, 2013).
The cost of implementing MACT will be high but the costs of climate change are even higher. The cost to implement the technology to meet these standards would be roughly 10.9 billion dollars per year for energy companies that are forced to comply (Bipartisan, 2013). This money would be made up by increasing utility for all customers of companies affected. The EPA estimates that rule would result in an electricity price increase of 3.7 percent and natural gas prices would increase by an average of 0.6 to 1.3 percent (Bipartisan, 2013). This would mean the average natural gas customer would see their yearly bill increase by between $5.95 and $12.90 and the average yearly electrical bill would increase by $49.98 (EIA 2016; Shannon, 2016). By increasing the prices of their customers the companies would be left with a small fraction of the actual cost of the technology and would therefore not have to take on such a financial burden.
The information provided has given use concrete examples and facts about the amount of pollution that’s being emitted into the earth’s atmosphere from fracking. We need to understand that we need to find a way to make natural gas the great clean energy source that is wanted by many people. Some methane emissions are essential to regulate because of their threat to climate change now and in the future, as we look more for the use of natural gas energy. By understanding the negative impacts of the extraction of natural gas is very important to know how we need to fix the problem of fracking to make fracking clean er and less pollutant. Overall we need to take some emissions present and reduce them to produce natural gas the green energy that is supposed to be. We have seen significant improvements in Colorado act to clean up the methane emissions from fracking.
AUTHORS
Andrea Vázquez – Animal Science
Noah Marchand – Environmental Science
Shawn MacDonald – Geology
REFERENCES
Allen, D. T., Torres, V. M., Thomas, J., Sullivan, D. W., Harrison, M., Hendler, A., . . . Seinfeld, J. H. (2013). Measurements of methane emissions at natural gas production sites in the United States. Proceedings of the National Academy of Sciences, 110(44),
17768-17773. doi:10.1073/pnas.1304880110
Alvarez, R. A., Pacala, S. W., Winebrake, J. J., Chameides, W. L., & Hamburg, S. P. (2012). Greater focus needed on methane leakage from natural gas infrastructure. Proceedings of the National Academy of Sciences of the United States of America, 109(17),
6435–6440. http://doi.org/10.1073/pnas.1202407109
Bipartisan. (2013, February 06). Assessment of EPA’s Utility MACT Proposal. Retrieved from: https://bipartisanpolicy.org/library/assessment-epas-utility-mact-proposal/
Bilgen, S. (2014). Structure and environmental impact of global energy consumption. Renewable and Sustainable Energy Reviews, 38(Supplement C), 890-902. doi:10.1016/j.rser.2014.07.004 British Columbia. Conventional versus unconventional oil and gas. Retrieved from: https://www2.gov.bc.ca/gov/content/industry/natural-gas-oil/petroleum-geoscience/pet-geol-conv-uncon
Brown, J. (2014). Production of natural gas from shale in local economies: A resource blessing or curse? Economic Review, 1-29. Retrieved from https://EconPapers.repec.org/RePEc:fip:fedker:00005
Caulton, D. R., Shepson,P. B., Santoro, R. L., Sparks, J. P., Hogarth, R. W., Ingraffea, A. R., .. . Miller, B. R. (2014). Toward a better understanding and quantification of methane emissions from shale gas development. Proceedings of the National Academy of Sciences, 111(17), 6237-6242. doi:10.1073/pnas.1316546111
Energy Information Administration. [EIA]. (2016). 2016 Average Monthly Bill- Residential. [Data file]. Retrieved from:https://www.eia.gov/ electricity/sales_revenue_price/pdf/table5_a.pdf
Energy Information Administration. [EIA]. (2017). Annual Coal Report. Washington, DC: U.S. Energy Information Administration.
Energy Information Administration. [EIA]. (2017). Annual Energy Outlook 2017. Washington, DC: U.S. Energy Information Administration.
Energy Information Administration.[EIA]. (2017). How much carbon dioxide is produced from burning gasoline and diesel fuel? Retrieved from https://www.eia.gov/tools/faqs/faq.php?id=307&t=9
Energy Information Administration. [EIA]. (2017). How much carbon dioxide is produced when different fuels are burned. [Table]. Retrieved from https://www.eia.gov/tools/faqs/faq.php?id=73&t=11
Environmental Protection Agency. [EPA]. (1994). Plugging and Abandoning Injection Wells United States Environmental Protection Agency Region 5 Guidance #4. Chicago, IL: Environmental Protection Agency.
Environmental Protection Agency. [EPA]. (2016). Sulfur Dioxide Basics. Retrieved from https://www.epa.gov/so2-pollution/sulfur-dioxide-basics#effects
Environmental Protection Agency. [EPA]. (2017). Inventory of U.S. Greenhouse Gas EmissionS and Sinks. (EPA Publication No. EPA 430-P-17-001). Washington, DC: U.S. Environmental Protection Agency
Howarth, R., Santoro, R., & Ingraffea, A. (2011). Methane and the greenhouse-gas footprint of natural gas from shale formations. Climatic Change, 106(4), 679-690. doi:10.1007/s10584-011-0061-5
Ingraffea, A.R., Wells, M.T., Santoro, R.L., & Shonkoff, S.B.C,. (2014). Assessment and risk analysis of casing and cement impairment in oil and gas wells in
Pennsylvania, 2000—2012. Proceedings of the National Academy of Sciences of the United States of America, 111(30), 10955-10960. doi:10.1073/pnas.1323422111 IPCC, 2013: Climate Change 2013: The Physical Science Basis. Contribution of Working
Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Stocker, T.F., D. Qin, G.-K. Plattner, M. Tignor, S.K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex and P.M. Midgley (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 1535 pp, doi:10.1017/CBO9781107415324.
IPCC, 2007: Summary for Policymakers. In: Climate Change 2007: The Physical Science
Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change [Solomon, S., D. Qin, M. Manning, Z. Chen, M. Marquis, K.B. Averyt, M.Tignor and H.L. Miller (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA.
Jacoby, H.D., F. O’Sullivan and S. Paltsev (2012): The Influence of Shale Gas on
U.S. Energy and Environmental Policy. Economics of Energy & Environmental Policy, 1(1): 37-51
Karion, A., Sweeney, C., Pétron, G., Frost, G., Michael Hardesty, R., Kofler, J., . . . Conley, S.
(2013). Methane emissions estimate from airborne measurements over a western united states natural gas field. Geophysical Research Letters, 40(16), 4393-4397. doi:10.1002/grl.50811
Kissinger, A., Helmig, R., Ebigbo, A., Class, H., Lange, T., Sauter, M., . . . Jahnke, W. (2013). Hydraulic fracturing in unconventional gas reservoirs: Risks in the geological system, part 2. Environmental Earth Sciences, 70(8), 3855-3873. doi:10.1007/s12665-013-2578-6
Kroh, K. (2013, November 19). Colorado to crack down on methane emissions from fracking. Retrieved from:http://grist.org/climate-energy/colorado-to-crack-down-on-methane-
emissions-from-fracking/
Machol, B., & Rizk, S. (2013). Economic value of U.S. fossil fuel electricity health impacts. Environment International, 52(Supplement C), 75-80. doi:10.1016/j.envint.2012.03.003
Magill, B. (2014, July 1). Fracked Oil, Gas Well Defects Leading to Methane Leaks.
Retrieved from: http://www.climatecentral.org/news/shale-gas-well-defects-methane-leaks-17701
Manfreda, J. (2015, April 14). The origin of fracking actually dates back to the Civil War. Retrieved from: http://www.businessinsider.com/the-history-of-fracking-2015-4
Marmaduke, J. (2016, May 12). Colorado oil and gas unaffected by new EPA methane rules. Retrieved fromhttps://www.usatoday.com/story/news/2016/05/12/epas-methane-rule -mirrors-colorado-regulations/84284706/
Nacamulli, M. (2017, July 13). How does fracking work? [Video file]. Retrieved from https://www.youtube.com/watch?time_continue=100&v=Tudal_4x4F0
Ogburn, C. S. (2014, February 25). Colorado First State to Limit Methane Pollution from Oil and Gas Wells. Scientific American. Retrieved from https://www.scientificamerican.com/article/colorado-first-state-to-limit-methane-pollution-from-oil-and-gas-wells/
Ophardt, C. E. (2013) Carbon Dioxide and Fossil Fuels [PowerPoint Slides]. Retrieved from Virtual Chembook Web site: http://chemistry.elmhurst.edu/vchembook/globalwarmA4.html
Schneising, O., Burrows, J. P., Dickerson, R. R., Buchwitz, M., Reuter, M., . . . Bovensmann, H. (2014). Remote sensing of fugitive methane emissions from oil and gas production in North American tight geologic formations. Earth’s Future,
2(10), 548-558. doi:10.1002/2014EF000265
Shannon, C. (2016, August 20). Utility Bills 101: Tips, Average Costs, Fees, and More. [Blog post]. Retrieved from http://www.move.org/blog/utility-bills-101 Sovacool, B. K. (2014). Cornucopia or curse? reviewing the costs and benefits of shale gas hydraulic fracturing (fracking). Renewable and Sustainable Energy Reviews, 37(Supplement C), 249-264. doi:10.1016/j.rser.2014.04.068
Turner, A. J., Jacob, D. J., Benmergui, J., Wofsy, S. C., Maasakkers, J. D., Butz, A., . . . Biraud, S. C. (2016). A large increase in U.S. methane emissions over the past decade inferred from satellite data and surface observations.Geophysical Research Letters, 43(5), 2218-2224. doi:10.1002/2016GL067987
Union of Concerned Scientists. [UCS] Shale gas and other unconventional sources of natural gas. Retrieved from: http://www.ucsusa.org/clean-energy/coal-and-other-fossil-fuels/shale-gas-unconventional-sources-natural-gas#.WiYtShiZNE6
West Virginia Department of Environmental Protection. (2014) MACT NESHAP Standards. Retrieved from http://dep.wv.gov/daq/Air%20Toxics/Pages/MACTNESHAPStandards.aspx
Nova88 Slot
Nova88
info slot gacor hari ini