The last two weeks have been thin on science. I have continued as I must with seed harvesting and cell passaging but there is little to say about these repetitious chores. However, I did have a two-hour stint on the scanning electron microscope. Previously (Jan 3 and Jan 24), I wrote about my collaboration with Joseph Hill (at Penn State) to examine the structure of the secondary cell wall of a set of mutants he made. A few months ago, we worked out a nice technique to image the fiber cell walls in his material, but three weeks ago I found out that, for the mutants, the method fell on its face. While the fiber cell walls in the wild type were clean as a whistle (really? – aren’t whistles blown by sticking them in one’s mouth?), in the mutants they were covered by glop. Ick.
We decided to test procedures for glop removal. Joe sectioned the mutant that has the most extreme phenotype and then treated the sections with bleach or chlorite. The former is good old Clorox and relies the fact that bleach will do to cytoplasmic debris (the presumptive glop) just what it will do to mold, spores, and all sorts of pigment molecules. Chlorite is a variation on the bleach theme and is a reagent known to degrade a variety of organic materials but not so much cell walls, because of their toughness. He also included a treatment where sections were incubated for a while in detergent because this too has the potential to clean up debris, and of course a good old control treatment (that is, the basic method, included to make sure that “cell walls covered by glop” is a recurring feature of the preparation).
I had a look a week ago, Thursday. The cell walls of fiber cells in the control looked as glop-fuscated as ever. Glop is bad but reproducibility is good. The detergent changed things to little if any extent, and this was not terribly surprising because the detergent used is fairly weak. However, here is an example of how fiber cell walls looked in sections that were treated with bleach:
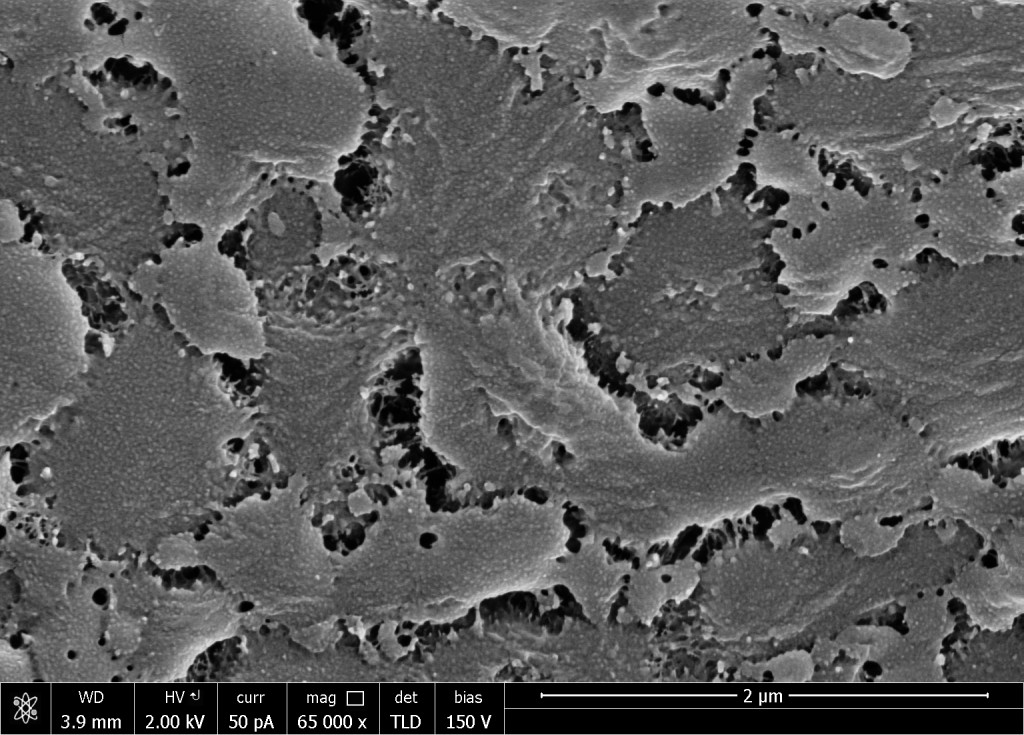
Seeing this brought me up short. I started looking more closely. To be sure, in the control sections, fiber cell walls were frequently hidden by what resembled cytoplasm. There were structures the size and shape of organelles (nuclei or plastids) and a lacy network that is probably a coagulated mass of membranes and cytoplasm. Fine. But through the holes in this lacework, the cell wall looked like in Figure 1, as it did in a few cells that had none of the lacey debris whatsoever. In the bleach or chlorite treated sections, there was far less of the lacey stuff and the fiber cell walls were all more or less like Figure 1. I found no instance where this material had lifted away to reveal a fibrous-looking cell wall.
Could the mutant fiber cell walls really look like this? Or is this some kind of super glop that is more tenacious than the lacey network? Fact or artifact? Arguments for both assertions exist.
On the fact side, I have looked at myriad cell walls over the years and they have always looked fibrous. Nevertheless, their fibrillar appearance probably depends on their containing abundant cellulose, which is thick, long, and linear, and likely to template a cell wall architecture that is inevitably fibrous. Well, surprise! Joseph’s mutants are low in cellulose. Perhaps with little cellulose, the remaining cell wall polysaccharides take on a gluey glopy nature? Figure 1 looks like some inorganic structure, Styrofoam or a complex mineral, and these forms might be similar to what cell wall compounds would form in the absence of the mechanically dominant cellulose.
On the artifact side, the preparation involves incubating pieces of stem in a chemical fixative for 15 hours or so, and then incubating it for another 18 to 20 h in ‘cryo-protectant’, a viscous solution of a proprietary nature designed to minimize ice crystal damage upon freezing and also to provide an embedment so that the samples can be sectioned while frozen (on a “cryostat”). It could be that the fixative is cross-linking cytoplasmic remnants to the cell wall or that the cryo- protectant solution sticks tenaciously to the wall. While the bleach and chlorite treatments we tried were supposed to chew threw such shrouds, there was enough obviously cytoplasmic debris remaining in both of these treatments that it seems neither was particularly aggressive.
To settle the matter, you guessed it, we need more tests. Joseph and I decided to compare the following: control (our usual method which involves overnight fixation followed by overnight incubation in cryo-protectant); no fixation – but overnight cryo-protectant; and, no fixation with ‘instant’ cryo-protectant; and all three of these will be treated both without bleach and with twice the concentration used previously. The ‘instant’ means that the stem segments will be immersed in the cryo-protectant and frozen immediately. We wondered whether this would allow sectioning at all but happily Joseph found a few days ago that it does. The cryo-protectant has a consistency somewhere between honey and tar; in the ‘instant’ cryo treatment, the segments of stem are frozen within moments of being introduced and they are open only at their cut ends; therefore, this should effectively let us look at fiber cell walls that have had little or no direct exposure to the cryo-protectant. In this way, if either the fixation or the cryo-protectant is messing the sections about (causing artifacts) then we should find that out. In the meantime, place your bets here!