… bit me. Of course, they always do. Examining an assumption reveals either that it is ok, to a first approximation, or that it isn’t; in the first case, the conditions are accepted as a reasonable compromise and, in the second case, conditions are changed. Without any check, unfortunate conditions come along for the ride, hidden under the seat. This past week, a hitchhiking assumption stumbled out of the car for a pee.
The assumption enters the story of how to stain cellulose in plant roots. I have written (here and here) about how a region of the root repels the cellulose stain, fast scarlet. This region exerts no such repulsion on another stain, calcofluor white; but, working with calcofluor requires short wavelength blue light (405 nm), a wavelength likely to damage the liquid-crystal based polarizing elements on which my method depends.
So far, I have been checking out the staining on an ordinary, wide-field epifluorescence microscope. Such a microscope is right around the corner from my bench. Now, the time had come for me to check staining on the confocal. With its laser light and sensitive detectors, this instrument could well show staining in the so-called dark zone, where my eyes looking through the wide-field microscope see nothing. I have not yet been able to get the software running that I need to do polarized fluorescence on the confocal but I had gotten trained on the instrument. And for checking the staining, regular confocal microscopy will be fine.
And so I checked. I examined two slides: one had roots stained with calcofluor and the other had them stained with fast scarlet. With the calcofluor, I learned that I could turn the laser power down as far as the machine would let me (to 0.2%) and still get crisp images without straining. [Sorry! I wish I could post one here but I don’t have access to the cloud storage space from home, only from on campus.] Maybe 0.2% is low enough to spare the liquid crystals, I will find out. But for the fast scarlet, I needed the laser turned up to around 10% and the gain cranked way up too. A noise fest, although with slow scans and image averaging, I got serviceable images. This is good, it means the region does take up some stain. But the amount is limiting. For all I know, that much green laser power would not be good for the liquid crystals. Anyway, the minimal staining will slow down the workflow and lower the quality of the results.
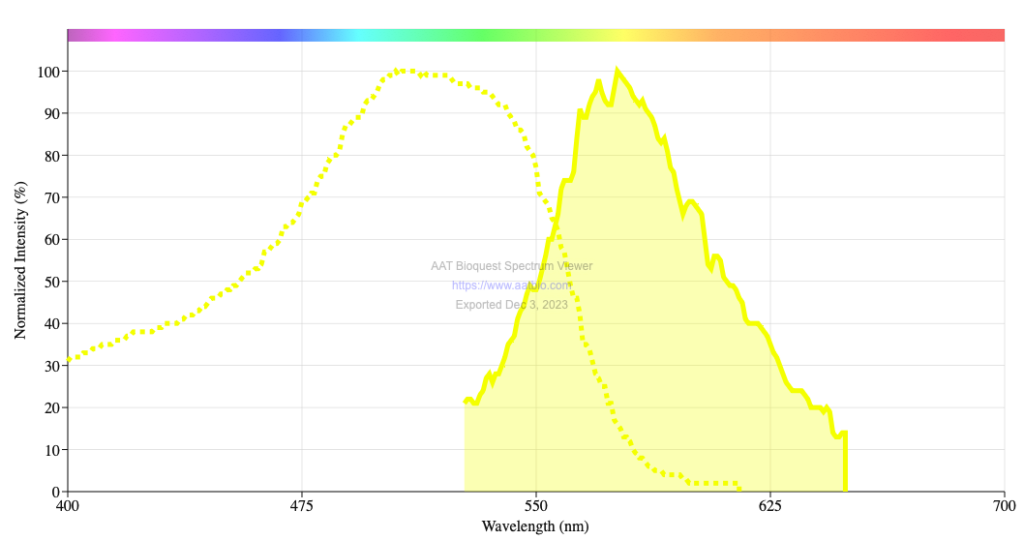
Figure 1. Fast scarlet excitation (dotted yellow line) and emission (solid yellow area) spectra. Spectra from the AAT Bioquest Spectrum Viewer.
Back in my office, I looked up the excitation spectrum for fast scarlet. Holy Goalie! The peak wavelength is 510 nm (Fig. 1). On the confocal, I have been exciting fluorescence with 561 nm. Why? Well, say hello to my unexamined assumption. I’d prefer you to snarl at it but you’d only be snarling at me. Turns out, in my previous work with fast scarlet at the Marine Biology Laboratory at Woods Hole, I used 561 nm for no other reason than that was the wavelength I was using. With the material I was imaging at that time (plant tissue culture cells and conical shaped cells on a flower petal), imaging at 561 nm was fine. The material stained well, dye was not limiting. I never checked whether 561 nm was the best laser line to use.
OK, better late than never. The LSM 780, the confocal I am working with here, is supposed to have a laser line at 514 nm, which would be brighter (Fig. 1). But the laser that provides light at 514 nm, an argon laser, is broken. A new argon laser is expensive; currently, the facility has no plans to replace. I might be stuck with staining at the limits of visibility. At the very least, I can make sure that the detector collects light as close to 561 nm as possible. But more exotic schemes are brewing…
Hiya Tobias, Sarah here! Greetings from Wisconsin. It’s my understanding that lasers are already polarized, and so I’m wondering about the function of the “liquid crystals” ? Our 780’s argon ion is in good working order, so you are always welcome to visit us in Madison with your interesting sample 🙂
Hi Sarah! Yes, the laser is polarized. What the liquid crystals do is allow the polarization state to be modulated. The protocol I am working with will collect four images, with linear polarization oriented at zero, 45, 90, and 135 degreees. From those four images, the average orientation of the dipole and their degree of anisotropy can be calculated at each pixel. This method was worked out by Rudolf Oldenbourg and colleages at MBL. When I get the system set up, I’ll be blogging about it. But in the meantime, I can send references if you are keen.
Nifty, thanks for the info, sounds like a useful method. I’ll let the suspense build until you blog more about it. Best of luck with your experiments! ðŸ€
Thanks. Hoping the wait won’t be tooooo long!